Model No.: YY-QR-9.0
Support customization: Oem, Odm
place of origin: China
Certification: CE
Cap: ABS
Detection principle: COVID-19 Antigen Saliva
Classification: Imaging Diagnostic Equipment
Type: Virus Rapid Test Kit
Features: Real Time Rapid Test
Packaging: carton ,single package
Productivity: 100000pcs/day
Brand: Yongyue or customized
Transportation: Ocean,Land,Air,Express
Place of Origin: China
Supply Ability: 100000pcs/day
Certificate: ISO13485
HS Code: 3926909090
Port: Shanghai Port
The COVID-19 China self corona test kit is a lateral flow immunoassay intended for the preliminary screening and qualitative detection of nucleocapsid protein antigen from severe acuterespiratorysyndrome coronavirus SARS-CoV-2which causesthe Coronavirusdisease(COVID-19),in direct saliva sample from individuals suspected of coVD-19 by their healthcare provider within the first seven days of symptom onset.The COVID-19 Antigen Saliva Test kit is for professional use only and is intended to be used as an aid in the diagnosis of SARS CoV-2 infection.
SARS-CoV-2is anenvelopedsingle-stranded RNAvirus ofthe Baenus COVD-19 is an acute respiratory infectious disease. People are generally susceptible.Currently. the patients infected bytheSARS-CoV2are the main source ofinfection:asvmptomatic infected peope car also be an infectious source Based on thecurrentepidemiologica investigation, the incubation period is 1to 14 daysmostly 3to 7 days The main manifestations include feverfatigue and drycough.Nasa congestion, runny nosesore throatmyalgia and diarhea are found in a few cases.
Results are for the identification of SARS-CoV-2 nuceocapsid protein antigen. The SARS-CoV-2 antigen is generally detectable in upper respiratory specimens during the acute phase ofinfectionPositive results indicate the presence of SARS-CoV-2viralantigenshowever cinicai corelation with patient history and other diaanostic information is key to determine status of infection.Positive results don't preclude bacterialinfection or co-infection with other yiruses.eaatveresuts To patients with symptom onset for more than seven days should be treated as presumptive and confirmation with a molecular assayand patient management may be performed according to local regulations as needed.
PRINCIPLE
The COVID-19 self corona test kit is animmunochromatographic membrane assay that uses highly sensitive monoclonal antibodies to detect the nucleocapsid protein of SARS-CoV-2 antigen in direct human saliva specimens. The corona kit test strip contains colloidalgold conjugated particles with monoclonal antibodies against the nucleocapsid protein of SARS-CoV-2. The secondary antibodies for nucleocapsid protein of SARS-CoV-2are coated on the membrane
When the saliva sample completed absorbed by the absorbent padthe conjugates immobilized in the reagent pad will be dissolved and migrate along with the sample.If SARS-CoV-2 antigen is present in the sample,a complex will be formed between the anti-SARS-CoV-2 conjugate and the virus will be captured by the specific anti-SARS-CoV-2 monoclonal antibodies coated on the test line(Tregion.Absence of the test line indicates a neaative result An internal procedural control is included in the assay, in the form of a colored line appearing in the control line(C) region,indicating that the proper and sufficient volume of specimen has been added and membrane wicking has occurred.
SPECIMEN COLLECTION
1.Take out the test pen from the package.Place on a flat horizontal and
clean surface.
2.Unfold the lid from test pen and directly place tip of the absorbent pad
beneath tongue. Press the absorbent pad firmly with tongue to obtain saliva specimens for 2-3 minutes until presence of a reddish color at the lower edge of the test membrane in the result window.
3.Remove the test pen from mouth and cover the lid. Place it on the flat.
horizontal and clean surface, and start timing
4.Read test result at 15 minutes. Do not read result after 30 minutes.
INTERPRETATION OF RESULTS
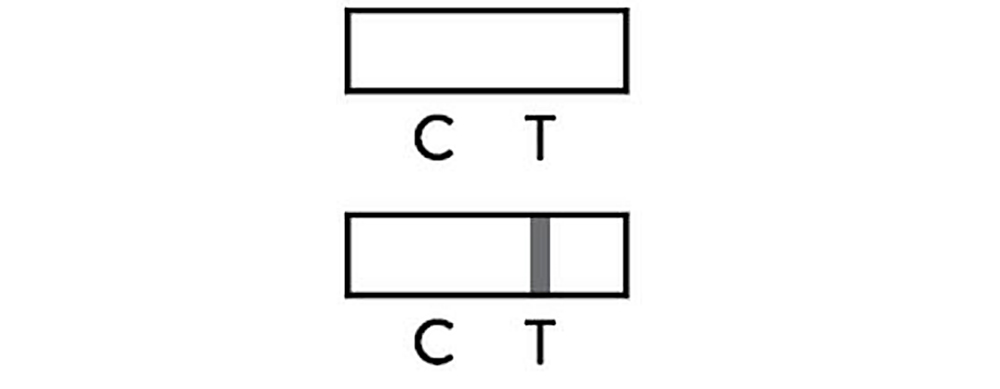
Negative
The presence of only control line(C) and no test line(T) within the result window indicates a negative result or the antigen concentration may be lower than limit of detection.
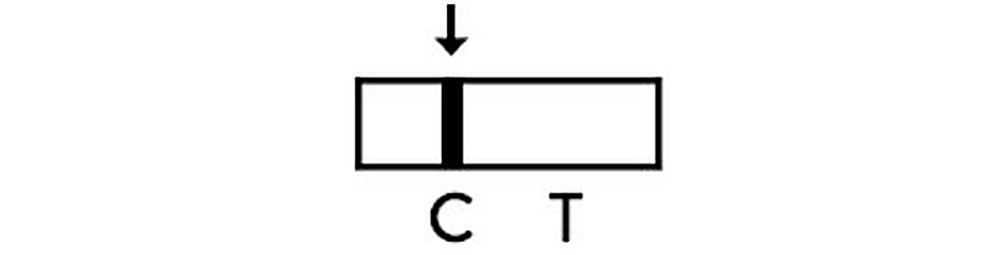
Positive
The presence of two lines as control line(C) and test line(T) within the result window indicates that SARS-CoV-2 antigen has been detected and test result is positive
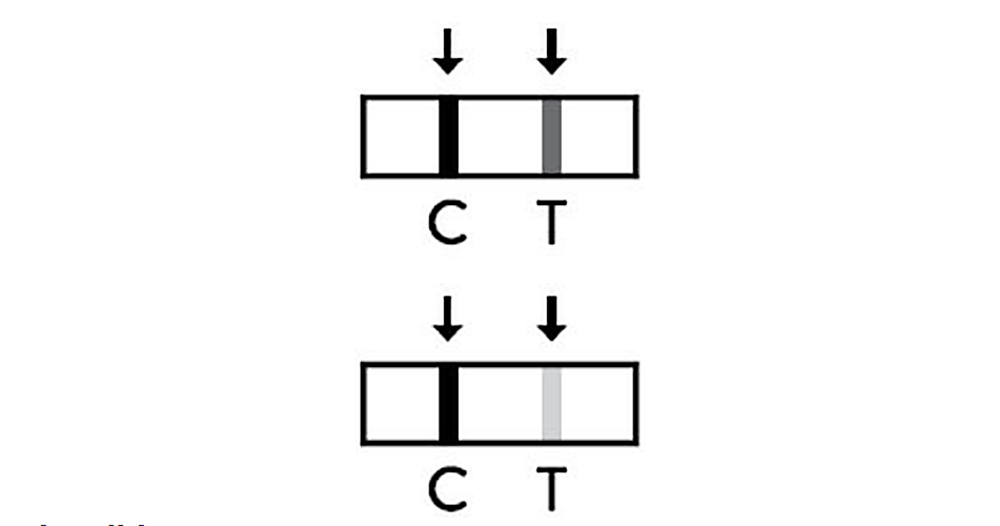
Invalid
If the control line(C) is not observed the result window after performing the test test result is considered as invalid regardless of whether there is test line (T) or not. It is recommended to perform the testing again using a new test pen.
Yong Yue Medical Technology(Kunshan) Co.,Ltd Copyright © All rights reserved Privacy Policy site map sitemap.html